Nuvaxovid
Nuvaxovid-rokote sopii lähes kaikille aikuisille. Like the Novavax vaccine side effects were more.

Ema Plans Anaphylaxis Label On Novavax Covid 19 Vaccine
Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre.
. Novavax Announces Shipments Of Its. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. 1 day agoAround 7000 doses of Nuvaxovid have already been administered in Sweden.
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. 16 fever including 14 severe cases. Nuvaxovid contains a version of a protein found on the.
The Summary of Product Characteristics is a description of a. Nuvaxovid contains a version of a protein found on the. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022.
Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. The addition of the saponin-based. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach.
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Det eftersom att data från.
On December 20 2021 the. As at 31 May four cases of adverse reactions were reported out of the 2792 doses of Nuvaxovid administered here at a 014 per cent incidence rate the report published on. As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus.
Qualitative and quantitative composition. This will enable us to start offering the Nuvaxovid. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further.
COVID-19 Vaccine recombinant adjuvanted 2. On August 19 2022 the Food and Drug Administration FDA authorized the Novavax COVID-19 Vaccine. Rokotteesta ei myöskään ole haittaa vaikka.
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. 88 experienced pain. Nuvaxovid contains a version of a protein found on the.
The agency said that younger people who had recently been vaccinated with Nuvaxovid had no. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. Beslutet är temporärt och gäller från.
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. Nuvaxovid dispersion for injection. 1 day agoPublicerad idag 0702.
This is a multidose vial. The first batch of Nuvaxovid is expected to arrive in. Name of the medicinal product.
1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen.
Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022
Novavax S Nuvaxovid Covid 19 Vaccine Granted Interim Authorisation In Singapore Cna

Eu Regulator Backs Use Of Novavax Covid Shot As A Booster
Novavax Covid Vaccine Nuvaxovid Gets Provisional Nod In New Zealand For Adolescents Aged 12 Through 17

Ec Expands Novavax Nuvaxovid Covid 19 Vaccine Approval

Novavax Nuvaxovid Covid 19 Vaccine Star Pharmacy

News Nuvaxovid Novavax Protein Based Covid 19 Vaccine Available On A Limited Basis In Germany Paul Ehrlich Institut

Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022

Novavax Requests Expanded Emergency Use Listing With Who For Nuvaxovid Covid 19 Vaccine For Adolescents Aged 12 Through 17 Eatg
Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022

Nuvaxovid Fifth Vaccine Against Covid Authorised In Eu Euractiv Com

Nuvaxovid Archives Drug Discovery And Development

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care
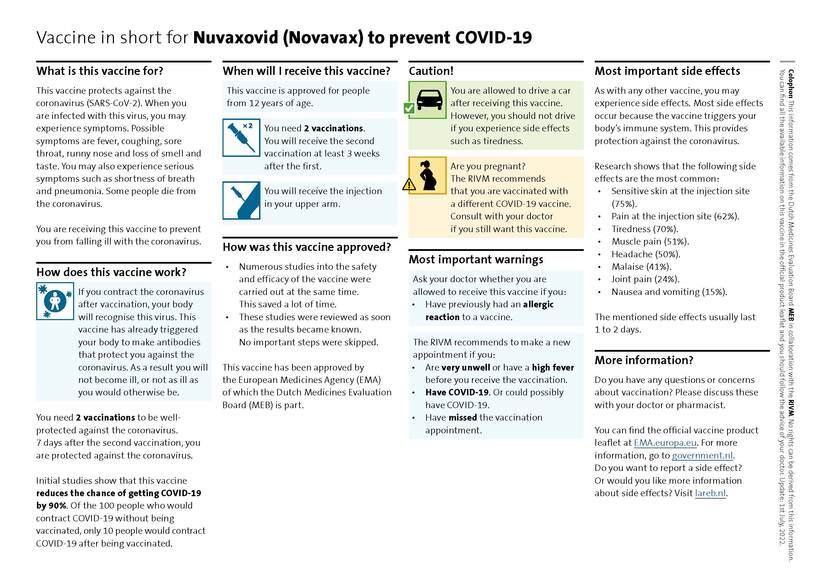
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News
Covid Vaccine Maker Novavax Drops After Cutting Sales Outlook 50 Nvax Bloomberg

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl